MassARRAY® SARS-CoV-2 Differentiation Panel
Agena has developed a SARS-CoV-2 detection system, assaying five viral targets in the N and ORF genes. This assay is granted Emergency Use Authorization in the U.S as well as CE-IVD approval.
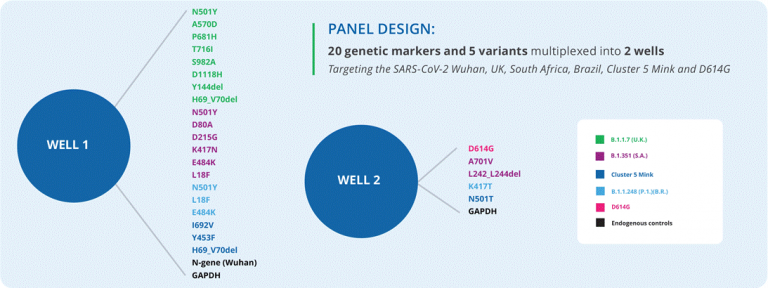
It is a high throughput and robust assay for the detection and differentiation of key SARS-CoV-2 Variants for use on the MassARRAY®System. Currently the panel differentiates the B.1.1.7 (UK), B.1.351 (South Africa), B.1.248 / P.1 (Brazil), Cluster 5/Mink (Denmark) and D614G lineages from the A.1 (Wuhan) lineage. New variants will be added continuously. The high-throughput MassARRAY System enables to cost-effectively process from hundreds up to thousands of samples per day with a single instrument.